The treatment of some physical and mental health related conditions is going digital and has evolved into its own field. These are therapies that use digital technology to not only prevent, but also manage and treat health conditions. Digital therapeutics (DTx) are now a part of the digital health field. They deliver therapeutic services and treatments directly to patients via software (usually web or mobile applications) that is clinically evaluated and proven to increase accessibility and effectiveness of healthcare. There’s also an FDA component and we’ll see fda digital therapeutics.
This emerging field has been steadily growing and gaining traction over the last few years. There is a lot to keep in mind when it comes to considering these types of therapies and in this post we will go over a list of fda approved digital therapeutics many aspects such as:
- What is (and what's not) Digital Therapeutics?
- Benefits of DTx
- FDA Regulations
- Uses cases for DTx
- Criteria for FDA regulations
- Examples of Digital Therapeutics
What is (and what's not) Digital Therapeutics?
Digital therapeutics deliver therapeutic interventions and services to patients through high quality software programs that are used to prevent, manage, or treat a disease or disorder. They can be used independently or in tandem with devices, medications, or other therapies to boost patient care and health outcomes.
Digital therapeutics have actually been on the market for about a decade, but there’s only been a few of them. Even less FDA approved digital therapeutics.
According to Insider Intelligence, it is expected that the Digital therapeutic market will be worth approximately $56 billion by 2025. Digital therapeutic vendors leverage their technological know-how to treat a myriad of conditions, which accounts for a large part of the US’ healthcare spending. The rushing need for therapeutic treatments combined with the after effects of the global pandemic accounts for the expansive growth of the digital therapeutic market.
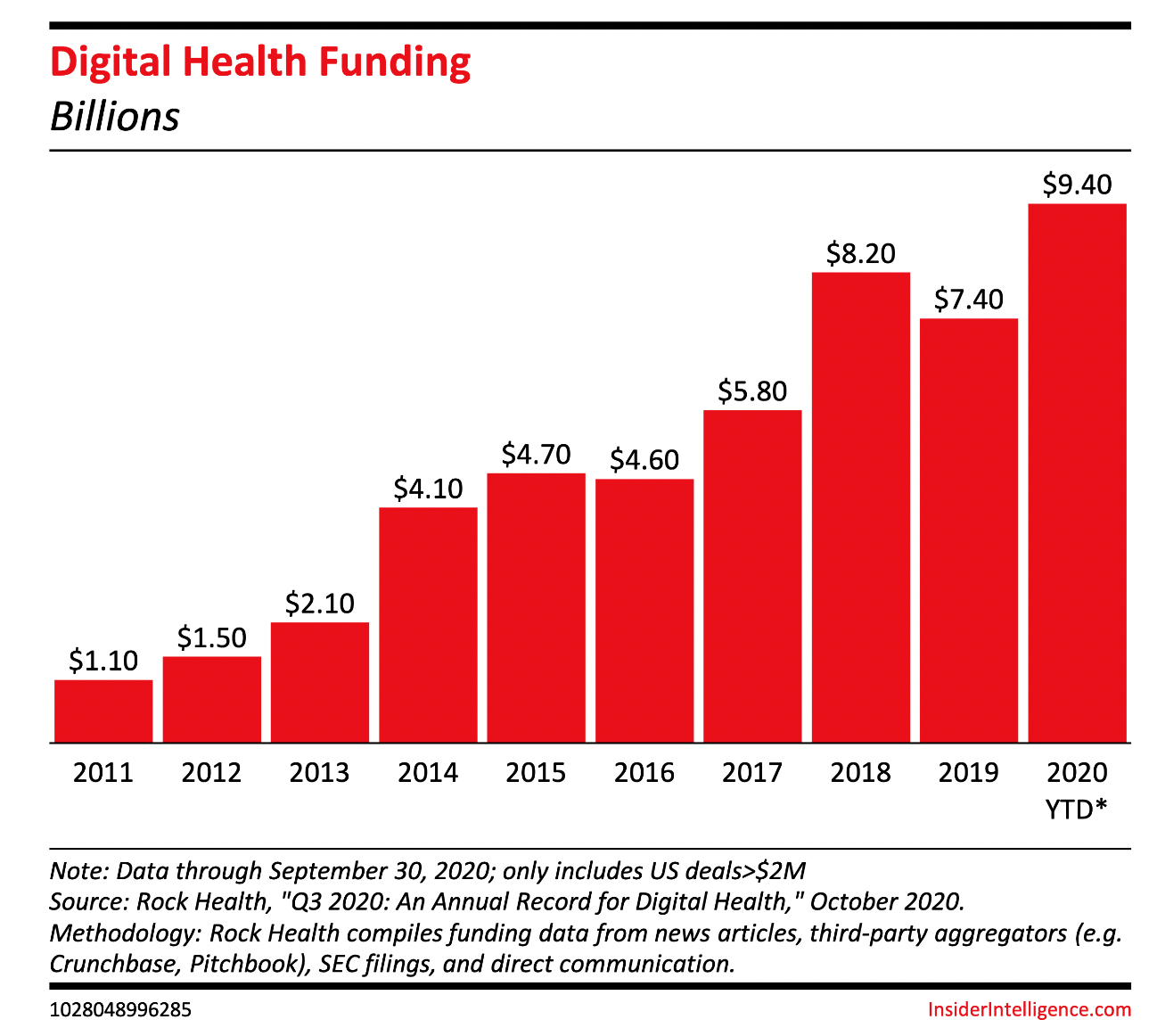
They predict that over the next few years, there will likely be more merger activity and closures among digital therapeutic companies. Pharmaceutical companies will more than likely start to acquire digital therapeutic providers, and large mergers and acquisitions are a key sign that the market is maturing and there is future growth.
Now let’s also talk about what’s not included in the digital therapeutics category. As we said DTx services treat, manage, and/or prevent a disease/disorder. So what we can rule out of the DTx field are consumer health and wellness applications. These could aid in sleep, nutrition, or mindfulness but they aren’t necessarily considered digital therapeutics. For example, apps that promote general health, wellness and fitness aren’t considered DTx. Neither are applications that solely inform, monitor, diagnose and/or provide physicians with insights. The difference with DTx products is that the user typically gains full access to the product’s content after they receive an authorization code (like a referral or prescription) from a physician, provider, employer, or other entity and enter it into the product.
In order to know if they are considered, or not, you could ask yourself the following questions:
- IS the intervention of this product used in the context of healthcare?
- Does the intervention treat, manage, or prevent a disease or disorder?
- Is software responsible for providing the intervention to the patient via a technology platform, or medical device?
In order to be considered as such they are required to be authorized by a regulatory body to support product claims of risk, efficacy, and intended use.
Benefits of DTx
As we saw above, DTx delivers therapeutic interventions to patients through high quality software. The purpose is to prevent, manage, or treat a disease/disorder. Digital therapeutics are expanding what was once globally achievable in healthcare through state of the art technologies that increase therapy accessibility and personalization for patients.
The plus with these types of therapeutic products is that they are evidence based and are clinically evaluated beforehand. They offer many benefits such as:
- Help lower healthcare costs
- Extend the physician’s ability to care for patients
- Evolve how patients understand, manage, and engage in their disorders
- They are easily accessible through devices the patient can own
- Offer at home convenience and privacy
- Help reach and deliver high quality care to underserved populations
- Optimize clinical and health outcomes
Since they are provided through high quality software, digital therapeutics are more readily available to people in remote areas. Hence why we say they help reach and deliver high quality care to underserved populations. These people have been historically underserved and now, thanks to digital therapeutic services, they have greater access to health care. It’s all about access, convenience and efficiency of care. They now have greater access to fda digital therapeutics.
Another great benefit is the reduction of overall healthcare costs for both patients and healthcare providers. By providing remote care, providers can cut healthcare costs and at the same time treat more people. This also translates to lower costs for the patients.
FDA Regulation
Digital therapeutics are considered and actually recognized as medical devices. They are, therefore, subject to various internationally recognized standards, as well as federal and local regulations. Digital therapeutic products need to also adhere to all core industry principles so they can demonstrate product quality, efficacy, and safety. They need to be patient centric, and adhere to privacy standards. FDA approved digital therapeutics aren’t the norm, and there aren’t a lot of approved therapies.
For example, a couple of years ago the Food and Drug Administration cleared a video game used to improve attention in kids diagnosed with ADHD, called Akili Interactive. This was the first time that, what’s considered a video game, was cleared for treatment by the agency.
Now, as the digital therapeutic market is growing exponentially, these companies have developed big ambitions. Ahead we’ll take a look at a list of fda approved digital therapeutics.
Criteria for FDA regulations
While fda digital therapeutics offer very unique opportunities within the healthcare product industry, the rapid progression of these technologies are causing numerous regulatory challenges. Despite these challenges, tech startups are shifting their focus onto healthcare and speeding up innovation in the health tech or digital health field.
Existing regulations for medical device software focus mainly on medical device software that is embedded in hardware medical devices. Regarding the regulatory point of view, digital therapies are encompassed in the Software as a Medical Device (SaMD) category. SaMD can be defined as software that is intended to be used for medical purposes that perform these purposes without being part of a hardware medical device. A medical device is described as software that is used for medical purposes but at the same time it does not act in the human body through immunological, pharmacological, or metabolic ways. Given that Software as a Medical Device has such unique features that go beyond what is a traditional medical device or other hardware, regulators from various countries recognized that there is a need to coincide on common principles for Software as a Medical Device that allows all stakeholders involved to promote safe innovation and at the same time protect patient safety.
In this day and age, medical device software is often able to carry out its medical intended purpose independent of hardware medical devices. It is important to correctly categorize SaMD in order to know their level of impact.
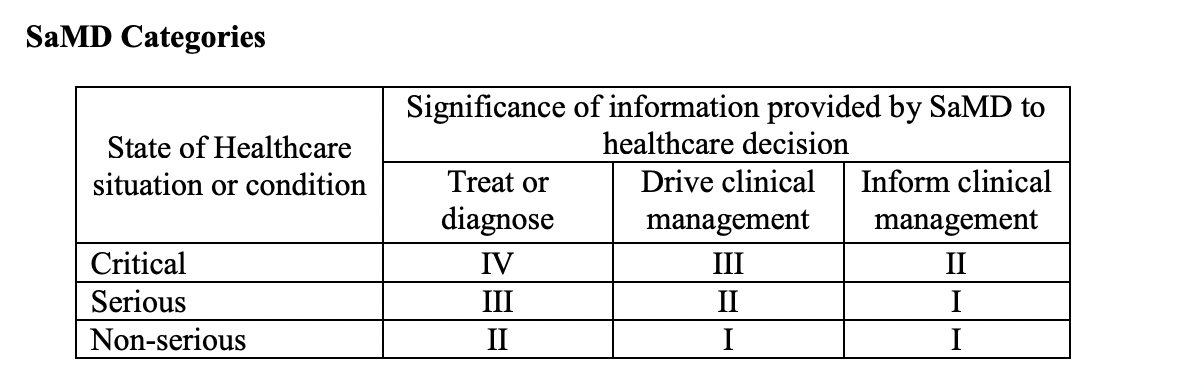
The four categories listed above (I, II, III, IV) are based on what level of impact the software has on the patient or on public health. This is whether the SaMD is meant to treat or diagnose, drive or inform clinical management in order to avoid death, long-term disability or other serious deterioration of health. The categories are in relative significance to each other with category IV having the highest level of impact, and category I the lowest.
Category I
It’s considered low impact and Category I software that is used to drive the clinical management of a disease or conditions in a non-serious situation.
It’s considered low impact and Category I software that delivers information to inform clinical management for a disease or conditions in a serious or non-serious situation.
Category II
It’s considered to be of medium impact and a Category II software that delivers info to treat or diagnose a disease or conditions in what is considered a non serious situation (or condition).
It’s considered to be of medium impact and a Category II software that delivers information used to drive clinical management of a disease or conditions in a serious situation or condition is a Category II and is considered to be of medium impact.
It’s considered to be of medium impact and a Category II software that delivers info to inform clinical management for a disease or conditions in what is considered a critical situation or condition is a Category II and is known to be of medium impact.
Category III
It’s considered to be of high impact and classified as a Category III software that delivers information to treat or diagnose a disease or conditions in a serious situation or condition.
It’s considered to be of high impact and classified as a Category III software that delivers information to drive clinical management of a disease or conditions that are in a critical situation or condition.
Category IV
It’s known to be of very high impact and classified as Category IV software that delivers information to treat or diagnose a disease or conditions in what is considered a critical situation or condition.
Examples of digital therapeutics
We have a list of FDA approved digital therapeutics, with the exception of Big Health whose products are CE-marked but not FDA cleared. Below we’ll list the examples of digital therapeutics.
Pear Therapeutics
They currently have three software products that are FDA-cleared: reSET which is a 90 day Prescription Digital Therapeutic (PDT) to treat substance use disorder, reSET-O an 84 day Prescription Digital Therapeutic (PDT) to treat opioid use disorder, and Somryst which provides cognitive behavioral therapy for insomnia.
They also provide an associated dashboard for providers to use during treatment. It displays information about the patient’s use of reSET, including patient reported substance use, lessons completed, compliance rewards, patient-reported cravings and triggers, and in person data inputs such as urine drug screen results.
Freespira
Is an FDA-cleared digital therapeutic used to reduce panic symptoms through guided breathing exercises and a respiration rate sensor.
AppliedVR
RelieVRx is an FDA-cleared at-home daily 7 minute immersive virtual reality (VR) pain treatment that is indicated as adjunctive treatment for chronic lower back pain. It’s clinically demonstrated to decrease pain severity in patients dealing with chronic lower back pain (CLBP).
Akili Interactive
Their product is EndeavorRx, which is an FDA-cleared action video game experience used for treatment of children with ADHD. The therapy is prescribed to improve attention function in children ages 8-12 years old as measured by computer-based testing.
Voluntis
Two FDA-cleared apps: Oleena is designed to help cancer patients to self-manage their symptoms, using evidence-based algorithms, to improve their treatment exposure, quality of life and ultimately increase survival. Their other DTx Insulia helps with insulin dose titration for Type 2 diabetes (it’s a prescription-only software medical device).
Big Health
They have two different therapies they offer. Sleepio provides revolutionary digital therapeutic for insomnia and has shown to be 74% effective. Daylight is a digital therapeutic that helps people gain control over their anxiety. These are CE-marked but not FDA cleared.
Digital therapeutics are not limited to chronic conditions like diabetes, or even just physical health. More and more are surfacing that tackle different conditions such as mental health. The most common application being, at the moment, the digital delivery of cognitive behavioral therapy (CBT) for anxiety disorders and depression.
The emergence of digital health is reshaping the entire healthcare system. Fda approved digital therapeutics are used for a wide range of diseases and disorders and provide new and advanced therapeutic options to patients, caregivers, and physicians in order to support or even enhance their current standards of care.




