The ideal agile software development partner for Medical Device companies
Innovative Software Solutions for Medical Devices
Since 2014, we’ve helped startups build and grow their digital products and services.
Our team of experienced software developers builds native and cross-platform mobile and web applications for medical devices. We integrate all kinds of wearables, IoT and custom hardware devices using technologies like NFC, bluetooth, wifi, and RFID.
We invest time in comprehending your specific needs to mold your concept into an ideal solution that can bring about significant change and transform patient care. We firmly believe that the key to success in medical device app development lies in meticulously crafting a solution that seamlessly aligns with your vision, fulfills patient needs, and meets market demands.
Our commitment revolves around designing digital products that deeply understand and prioritize the unique needs of patients. We place a strong emphasis on user-centric design when it comes to medical device software development. Our team is proficient in supporting you with usability studies, collecting user feedback, and refining your product's user experience to elevate its safety and efficacy.
Medical device app development involves leveraging agile methodologies in order to build accessible and user-friendly mobile and web apps. Since they are built for physicians and patients, it’s important to create solutions that are intuitive and easy to use, ensuring a seamless experience for everyone while taking the appropriate measures to protect sensitive patient information.
We create software that pairs with medical devices that are equipped with communication capabilities, allowing them to connect to other devices, networks, or systems for data exchange and interaction. We do this by using technologies such as NFC, bluetooth, wifi, and RFID.
to the medical device industry
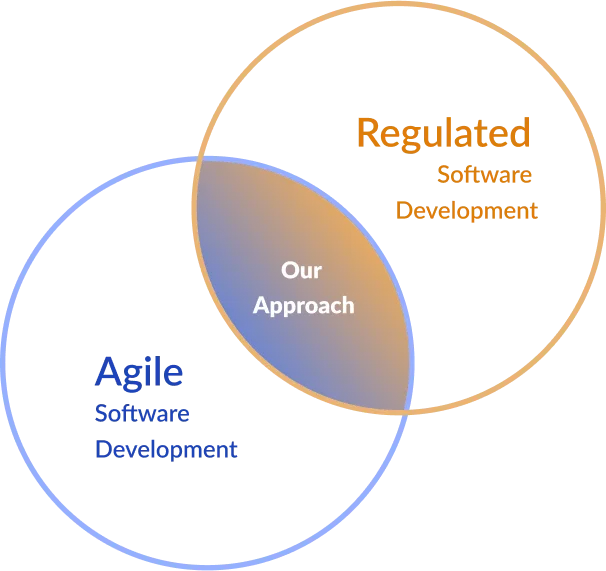
Agile medical device software development is an approach to creating software for medical devices that incorporates the principles and practices of Agile methodology while adhering to the strict regulatory requirements of the medical devices industry.
This approach is designed to improve the development process, enhance collaboration, and increase flexibility while ensuring the safety and efficacy of the medical devices.
Involves breaking the development process into small, manageable iterations or sprints. Each iteration typically lasts two to four weeks, during which a specific set of features or requirements is developed and tested.
Agile teams can include software developers, quality assurance professionals, and regulatory experts. This collaborative approach ensures that all aspects of medical device development, including compliance with regulations, are considered.


User feedback is critical in the development of medical devices. Agile methodologies emphasize early and continuous engagement with end-users to validate and refine the product throughout development.
Comprehensive documentation is essential for regulatory approval and quality control. Agile practices typically incorporate just-in-time documentation, meaning that documentation is produced as needed and kept up-to-date as the project evolves.
Rigorous verification and validation activities are integrated into the Agile process to ensure that the medical device software meets the specified requirements and functions safely and effectively.
Agile principles encourage continuous improvement through retrospectives at the end of each iteration. This process allows teams to identify issues and make necessary adjustments to improve both the development process and the product.


Agile medical device software development is an approach to creating software for medical devices that incorporates the principles and practices of Agile methodology while adhering to the strict regulatory requirements of the medical devices industry.
This approach is designed to improve the development process, enhance collaboration, and increase flexibility while ensuring the safety and efficacy of the medical devices.
Involves breaking the development process into small, manageable iterations or sprints. Each iteration typically lasts two to four weeks, during which a specific set of features or requirements is developed and tested.
Agile teams can include software developers, quality assurance professionals, and regulatory experts. This collaborative approach ensures that all aspects of medical device development, including compliance with regulations, are considered.

User feedback is critical in the development of medical devices. Agile methodologies emphasize early and continuous engagement with end-users to validate and refine the product throughout development.
Comprehensive documentation is essential for regulatory approval and quality control. Agile practices typically incorporate just-in-time documentation, meaning that documentation is produced as needed and kept up-to-date as the project evolves.

Rigorous verification and validation activities are integrated into the Agile process to ensure that the medical device software meets the specified requirements and functions safely and effectively.
Agile principles encourage continuous improvement through retrospectives at the end of each iteration. This process allows teams to identify issues and make necessary adjustments to improve both the development process and the product.

to work with medical devices
By staying current with privacy regulations and the latest data security standards, we work diligently to incorporate robust privacy measures into your software's design and development. This includes the implementation of appropriate protective measures to protect sensitive patient information and ensuring compliance with relevant standards such as the Health Insurance Portability and Accountability Act (HIPAA).
We can help integrate Electronic Health Records (EHR) with medical devices, facilitating the efficient flow of patient data for enhanced healthcare delivery, streamlined workflows, and improved patient care.
We understand the challenges that startups face when navigating the regulatory landscape. While physical medical devices are classified as I, II, and III, the FDA recognizes and aligns with the IEC 62304 standard to classify software as A, B or C. We are here to provide comprehensive assistance and support throughout the FDA process, to help ensure that your software meets the necessary standards.





